If you have ever opened the hood of your car and seen the green and white mixture on the terminals, you may have wondered why this happens. What can I put on the battery terminals of my car to prevent corrosion? What causes battery terminal corrosion?
Before any of this, I always keep this bad boy in my car. In case of a dead battery in my car or a friendly person, this guy comes in handy quite often.
Car batteries are susceptible to damage if not taken care of, which is why it’s so important to keep them clean and free of corrosion. If you do not take care of your battery, it can become damaged and eventually fail. To prevent this, clean your battery terminals often.
If you have a damaged or corroded battery terminal, it can cause your car to not start. If you have a damaged or corroded battery terminal, it can cause your car to not start. Various causes of corrosion of the battery terminals have been given.
There are ways to prevent and remove corrosion. But sometimes you have a lame battery and you need to put it down. If so, I love this one.
If you have ever opened the hood of your car and seen the green and white mixture on the terminals, you may have wondered why this happens.
Table of Contents
Repairing A Damaged Battery Terminal Cable
Repairing a damaged or corroded electrical connection is a hard job. The job can be made more difficult by the fact that the connection may have become contaminated with dirt or grime.
Releasing too much hydrogen gas happens when the battery is overcharged. You can tell this is the case because the electrolyte will turn brown and the electrolyte level will drop.
To get the job done, you’ll need to use a little bit of elbow grease and some good old-fashioned elbow grease. Try using a toothbrush to clean the connection, or use a wire brush to get the job done.
How Do I Prevent Battery Terminal Corrosion?
You can prevent battery corrosion by following these suggestions:
- You need to keep the electrolyte level at a safe level. The alternator converts electrical energy to mechanical energy. If the alternator is not working properly, it can cause your car to overheat and have a lot of trouble starting. If you have a faulty alternator, it can also cause problems.
- When the positive and negative terminals of a battery are connected together, they can become corroded. When the battery voltage is less than 3.3 volts, it is considered to be overcharged.
- Do not fill your batteries to the top. Hydrogen gas can react with other gasses to form corrosive compounds. To avoid this, always keep your car’s battery fully charged. Follow these simple steps to keep your car’s battery healthy.
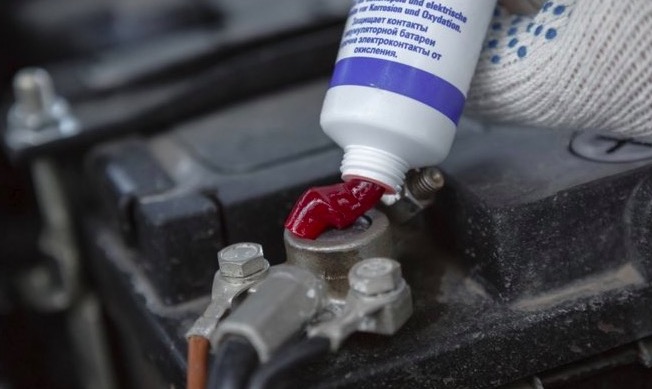
- NCP2 Battery Corrosion Preventative grease works well. It is a fancy silicone oil-based grease that doesn’t hurt anything. It’s good to have around.
Batteries can last for many years, but not all of them are created equal. The lifespan of a lithium-ion battery depends on many factors, such as how it is used, how it is stored, and how it is maintained. If the battery is not kept in good condition, it will eventually fail.
How To Clean Battery Terminal Corrosion
Wipe the terminals clean with a little petroleum jelly to prevent corrosion. The terminals can be protected from corrosion by applying a thin coating of petroleum jelly to the ends of the cables. Wipe the terminals clean every time you change the batteries.
To prevent corrosion from forming on the battery terminals, follow these steps. If you clean your batteries regularly, they will last a long time free of corrosion.
The reaction of the battery with the atmosphere produces hydrogen gas, which is harmless. If it forms on a positive terminal, it means that the battery is charging or discharging. Batteries are susceptible to corrosion if they are not kept clean. If you do not keep your batteries clean, they can become damaged.
Baking soda is a common home remedy for removing rust and corrosion from metal surfaces. Simply add a little baking soda to a glass of water, and you will have a solution that can be used to clean metal. You can also use a toothbrush to scrub the corrosion off of the metal.
If you want to protect the metal from further damage, you can put a thin layer of baking soda on the corrosion. When you add a little water to the electrolyte solution, you will notice that it begins to bubble.
The reaction between the water and the acid in the electrolyte will neutralize the acid and make it safe to handle. To neutralize the acid, you can repeat the process by adding a little more water.
Many soft drinks contain carbonic acid, which can cause discoloration of the teeth. You can use baking soda to remove the stain.
You will need baking soda and water to remove copper sulfate from the copper wires. Ensure the car’s ignition is turned off when you’re doing this. Then, you should gently pour the solution over the terminals and then scrub them thoroughly with a wire brush. After this, rinse the terminal with clean water, and then put it back into service.
Take a cup of warm water and add a pinch of salt, then add a few drops of lemon juice and a few drops of peppermint essential oil, stir well and let it stand for a few minutes, then strain it through a fine sieve. You can also soak a cotton ball in warm water for a few minutes and then squeeze it through a fine sieve.
You will need to use a solution of baking soda and water to get rid of the copper that has built up on your car’s electrical system. Turn off the engine and wait for the problem to go away.
There is a special battery terminal cleaning tool that is small, cheap, and easy to use. It comes in two parts that disconnect where you can use each side. The female side brushes away the big parts of corrosion on the battery terminal and a male part you can use to clean the cable terminal.
More Tips To Prevent Battery Terminal Corrosion
The best clamps for connecting the battery to the wire are made of copper, as they are not liable to corrode by themselves; but, when the battery is exposed to sulfurous gases, and the current passing through them, they form a deposit of copper sulfate, which corrodes the terminals.
Also, make sure your clamps are securely tightened. They cannot rotate by hand. That will make a secure connection that will not cause any problems in the future. Also copper is very pliable. It will morph very easily, so if you over tighten it you can easily rip the terminal rendering it useless.
You can also use brass. Brass is much cheaper and more readily available, but it is not as good as copper. But it may be more practical being an easier metal to use for auto applications. Make sure you know the difference. Copper has a deeper orange color and brass is more yellow:

Copper is a very good conductor, but it is not immune to corrosion. When you connect a car battery to a copper wire, you can cause corrosion in the battery terminals. If you see a white deposit on the battery terminals, it’s a sign that you need to change the battery.
Adding dielectric grease will cover the terminal connection and prevent air from reaching the bare metal, thus preventing most if not all corrosion:
Most quality clamps that connect the battery to the wires are made of copper. However, if the battery is leaking sulphuric acid, the copper will begin to corrode. The solution for this problem is to add a little copper sulfate to the battery, which will cause the corrosion to stop.
Main Takeaways – Battery Terminal Corrosion Causes
A lot of people have experienced battery terminal corrosion. This can be caused by a variety of factors, but one is the length of time it takes to charge your car or bike battery each day. If you are charging your battery for more than 12 hours every night, then this could cause corrosion on the terminals and lead to some serious problems with starting up your vehicle in the morning.
You may need to invest in an expensive charger that will allow you to set how long it charges for at different intervals throughout the day so as not to overcharge your batteries when they are sitting overnight without use.
These chargers also keep track of how many times per month you’ve charged them which is important because if you don’t know how much juice was put back into the battery, you could find yourself with a dead vehicle after sitting for a long period of time.
Batteries contain sulfuric acid which is what gives them their charge and powers your vehicle’s electrical system. This acid can eat through metals such as copper and steel over time which will make it harder to start your car in the morning.
If there is too much acid on the battery terminals then it could eat through the rubber coating, which will further corrode your car even more. This is why it’s very important to keep a small amount of acid on the battery posts at all times.
If you are experiencing corrosion after charging your vehicle overnight, you may need to adjust how you’re charging your vehicle. Most modern cars will tell you the specific interval at which they need to be charged by blinking on your battery’s indicator light.
You can also contact someone who works on batteries in order to get them tested and see if they are still any good for use after getting recharged on a more regular basis.
I hope this article will help you put a stop to any battery terminal corrosion problems you may be having with your vehicle. If the corrosion persists, the terminals may need replacing.
Thanks for reading and stay dirty